With organoids to model early growth, scientists employed a developing microscopy technology to observe that new neurons find it hard to reach their developmental goal.
Using an advanced microscopy approach, researchers at The Picower Institute for Learning and Memory at MIT have seen how newborn neurons struggle to attain their appropriate places in developed human brain tissue models of Rett syndrome, thereby generating a new understanding of how developmental deficits noticed in the patients’ brains with the disturbing disorder may emerge.
Mutations in the gene MECP2 are responsible for Rett syndrome—characterized by indications that include severe intellectual disorder and weakened social behavior. To attain fresh insight into the way the mutation impacts the beginning stages of human brain growth, scientist in the lab Mriganka Sur, Newton Professor of Neuroscience in MIT’s Department of Brain and Cognitive Sciences, raised 3D cell cultures known as cerebral organoids, or minibrains, with the help of cells from people with MECP2 mutations and compared them to otherwise alike cultures with no mutations.
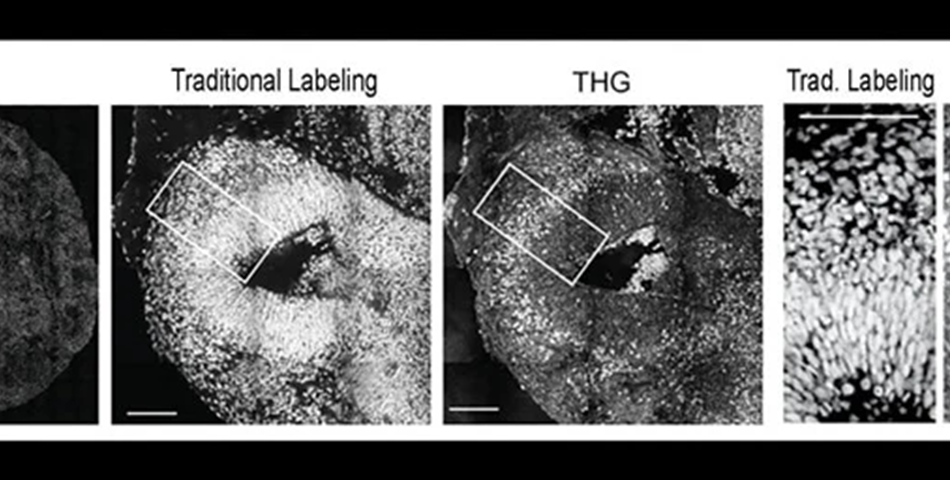
Then, the team headed by postdoc Murat Yildirim assessed the growth of each type of minibrain with an advanced imaging technology known as third harmonic generation (THG) three-photon microscopy.
THG, which Yildirim has assisted to create in Sur’s lab by collaborating with MIT mechanical engineering Professor Peter So, enables very high-resolution imaging deep into live and intact tissues without requiring to include any chemicals to label cells.
The new study was published in eLife. It was the first to employ THG to image organoids, without disturbing them virtually, Yildirim said. Earlier organoid imaging studies have needed employment of technologies that could not image throughout the 3D tissue, or methods that needed killing the cultures: either by cutting them into thin segments or clearing them chemically and labeling them.
Three-photon microscopy uses a laser, but Yildirim and So custom-made the lab’s microscope to not put any more power on the tissue than a cat toy laser pointer, which is less than 5 mW.
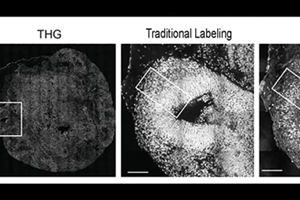
The researchers gained sufficient signal to attain label-free, intact imaging of fixed and live organoids even at low power. To verify it, they compared their THG images to images achieved through more conventional chemical labeling methods.
The THG system enabled tracking of the movement of newborn neurons as they migrated from the rim surrounding open spaces in the minibrains (known as ventricles) to the outer edge, which is analogous to the cortex of the brain directly.
They observed that the emerging neurons in the minibrains modeling Rett syndrome migrated gradually and in meandering paths when compared to the quicker motion in straighter lines as demonstrated by the similar cell types in minibrains with no MECP2 mutation. Sur stated the consequences of those migration deficits are consistent with what scientists, along with the scientists in the lab, have theorized to be occurring in Rett syndrome fetuses.
He is also the director of Simons Center for the Social Brain in MIT.
Tissues are imaged by THG without labels as they are very sensitive to alterations in the refractive index of materials, Yildirim stated. Hence, it resolves boundaries among biological structures, like cell membranes, extracellular spaces, and blood vessels.
Since neural shapes get modified while their development, the team could see the delineation clearly between the ventricular zone (an area surrounding the ventricles where the newborn neurons develop) and the cortical plate (an area where the mature neurons settle down). Also, it was very easy to resolve different ventricles and section them into separate regions.
Such properties enabled the scientists the possibility to observe that in Rett syndrome organoids, the ventricles were bigger and more in number and that the zones of ventricles, which are the rims surrounding the ventricles where neurons emerge, were thinner.
In live organoids, the researchers could track some of the neurons migrating toward the cortex in some days, clicking a new picture every 20 minutes, as neurons present in real growing brains would try to do.
They observed that Rett syndrome neurons attained only around two-thirds the pace of non-mutated neurons. The Rett neurons’ paths were also considerably wigglier. Together, the two differences meant that the Rett cells had hardly got half as far.
Yildirim, who will start his own lab as an assistant professor at the Cleveland Clinic’s Lerner Research Institute in September, stated that he has new insights depending on the results. He would like to image later in organoid growth to track the results of the sinuous migration. He also wished to know more about if particular cell types find it hard to migrate more or less, which can change the way cortical circuit’s function.
Yildirim also stated that he hopes to pursue advancing THG three-photon microscopy, which he perceives as having the capacity for fine-grained imaging in humans. It could be an essential benefit for people particularly as the imaging method can pass deep into living tissue with no need for artificial labels.
Along with Yildirim, Sur, and So, the other authors of this paper are Chloe Delepine, Danielle Feldman, Vincent Pham, Stephanie Chou, Jacque Pak Kan Ip, Alexi Nott, Li-Huei Tsai, and Guo-li Ming.
The National Institutes of Health, The National Science Foundation, the JPB Foundation, and the Massachusetts Life Sciences Initiative funded this research.
Source: The Picower Institute